| |
|
|
Septic shock |
|
|
|
Thursday, 14 October 2004 |
Click on each figure to increase it - If the enlarging does not function, check in your browser's option that 'javascript' is well activated.
Hemodynamic management of septic shock using echocardiography necessitates round-the-clock access to transesophageal echocardiography. Echocardiography should be done at least once a day, and also whenever it proves necessary, for example if blood pressure monitoring reveals new hemodynamic instability. The esophageal probe can be frozen and left in place pending initiation of suitable therapy so that a few minutes later the impact on the patient’s hemodynamic profile can be assessed. Echocardiography should be used systematically and repeatedly to identify the principal causes of circulatory insufficiency, which are often implicated in sepsis: hypovolemia, systolic dysfunction of the left ventricle, right heart failure, and vasoplegia (diagnosed by elimination when there is circulatory insufficiency but none of the other three causes is found by echocardiography).
1- Unmasking hypovolemia.This is the first step of the examination, given the modifications that hypovolemia can induce, notably in the parameters of systolic function of the left ventricle. We have demonstrated the reliability of the collapsibility index of the superior vena cava (SVC) in patients on assisted ventilation in deciding on blood volume expansion. For this a longitudinal view of the SVC must be recorded, by coupling motion mode (TM-mode) with two-dimensional mode so as to study SVC size variations during a respiratory cycle.
The collapsibility index is calculated as maximum diameter - minimum diameter / maximum diameter. 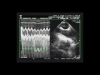 Figure 1 : The pressure signal in the airways appears in green on the screen Figure 1 : The pressure signal in the airways appears in green on the screen
Maximal and minimal diameters are indicated by the arrows. |
The SVC is subject to intra-thoracic pressure, and increase in this pressure on insufflation can, in the case of hypovolemia, result in a large diameter decrease or even collapse of the SVC (Film 1 , Film 2). A collapsibility index above 36% indicates the need for vascular filling (Film 3 which, if sufficient, will lead to significant increase in cardiac flow and decrease in SVC diameter variations during insufflation (Film 4).In the absence of an esophageal probe, or for an overview, echocardiography by the subxiphoid route can be done. It visualizes the inferior vena cava (IVC) and can be used to calculate the distensibility index, to decide whether or not to perform blood volume expansion. The distensibility index of the IVC is calculated as maximum diameter - minimum diameter / minimum diameter (Figure 2).
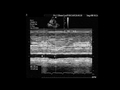 Figure 2 : E: Expiration, I: Inspiration Figure 2 : E: Expiration, I: Inspiration
Significant increase of IVC diameter related to tidal volume in a septic patient
|
Unlike the SVC, the IVC is not subject to intra-thoracic pressure, and so tends to increase in size at each insufflation, especially if hypovolemia is present (Film 5, Film 6). A distensibility index above 18% could indicate, in the presence of circulatory insufficiency, a need for blood volume expansion (Film 7, Film 8). This index is, however, less reliable than the collapsibility index of the SVC as it depends, among other things, on intra-abdominal pressure, which is the external pressure on the IVC. In certain conditions of high abdominal pressure, variations in IVC diameter can thus be attenuated. 2- Looking for severe left ventricular systolic dysfunction.Left ventricular systolic dysfunction is classically reported in septic shock. However, in contrast to the opinion of most intensivists, it is not rare. Even if the so-called hyperkinetic profile is predominant (Film 9), we have found a hypokinetic state in 35% of almost 200 patients with septic shock. We define a hypokinetic profile as a combination of a cardiac index below 3 l/min/m2 and of an LV ejection fraction (LVEF) below 40% (Film 10). So, LV systolic dysfunction should always be sought. This requires long-axis and short-axis views of the LV, ideally by transesophageal electrocardiography (TEE), but also sometimes by transthoracic electrocardiography (TTE). The long-axis view enables measurement of ventricular volumes and therefore of LVEF (LVEF = end-diastolic volume – end-systolic volume / end-diastolic volume) (Figure 3) (Film 1 and Film 7, first chapter). The short-axis view allows measurement of ventricular areas and therefore of the LV fractional area contraction, close to its ejection fraction (FAC = end-diastolic area - end-systolic area / end-diastolic area) (Figure 3) (Film 4 and Film 9, first chapter). 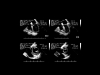 Figure 3 : Measurement of volumes and left ventricular area by TEE. Figure 3 : Measurement of volumes and left ventricular area by TEE.
ED: end-diastolic, ES: end-systolic |
Our therapeutic approach is systematized from echocardiography: in the presence of circulatory insufficiency, or of metabolic acidosis reflecting tissue hypoxia, an LVEF or an LVFAC below 40% systematically requires infusion of an inotropic agent (dobutamine usually, sometimes epinephrine) (Film 11). Echocardiography must always be repeated after initiation of infusion, to check treatment efficacy (Film 12). For TEE, the transesophageal probe is left in place a few minutes, while frozen. In intermediate situations where LV systolic function is moderately decreased (LVEF between 40 and 50%), the treatment should be done case by case. Systematic and regular echocardiography should be used to monitor left ventricular function on initiation of treatment with a pure vasoconstrictor like norepinephrine. We have already observed that norepinephrine, by increasing afterload on the LV, can unmask LV systolic dysfunction requiring addition of an inotropic agent. This is illustrated by Film 13, Film 14 et Film 15.  Figure 4 : Protocol for hemodynamic management of septic shock using TEE. Figure 4 : Protocol for hemodynamic management of septic shock using TEE.
LVEF : left ventricular ejection fraction. NEP : norepinephrine. Dobu : dobutamine. |
3- Looking for right ventricular failure.In certain situations, right ventricular (RV) systolic dysfunction may occur alone or combined and cause persistent circulatory insufficiency. This happens when there is intrinsic alteration of RV contractility, which was described in septic shock many years ago, or when there is acute cor pulmonale (ACP) due to severe acute respiratory distress syndrome (ARDS). So, in all cases in this third and last part of the examination, echocardiography should assess right ventricular systolic function. We never use RV fractional area contraction, calculated from a long-axis view of the LV (RVFAC = RV end-diastolic area – RV end-systolic area / RV end-diastolic area), because in our experience it is neither sensitive nor specific in the diagnosis of RV systolic dysfunction. On the other hand, and given the usual functional characteristics of the RV when there is low pressure circulation, any significant right ventricular failure is accompanied by dilation of the RV. This is therefore the principal parameter that we evaluate from a long-axis view of the LV which distinguishes the four cardiac chambers (Figure 5). Subcostal echocardiography reveals the IVC, which often seems dilated in right ventricular failure (Figure 6). RV dilation may be associated in certain circumstances, as ARDS, with paradoxical movement of the interventricular septum, indicating systolic overload of the ventricle. Right ventricular failure is then in keeping with acute cor pulmonale (ACP) (Film 16). 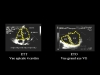 Figure 5 : Method of detecting RV dilation Figure 5 : Method of detecting RV dilation | 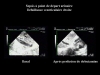 Figure 6 : Visualization of the IVC by the subcostal route in a female patient hospitalized for septic shock. IVC dilation, reflecting the right heart failure, is corrected by dobutamine infusion. Figure 6 : Visualization of the IVC by the subcostal route in a female patient hospitalized for septic shock. IVC dilation, reflecting the right heart failure, is corrected by dobutamine infusion. |
Apart from adaptation of assisted ventilation, which will not be discussed here, the treatment of right ventricular failure, partly or wholly responsible for circulatory insufficiency, depends on the state of LV systolic function. In practice, when RV failure is combined with marked LV systolic dysfunction, infusion of an inotropic agent is required (Film 17, Film 18). Otherwise, norepinephrine seems most effective (Film 19, Film 20). Lastly, detection of ACP is important, not only for ventilatory treatment, but also to circumvent certain mistakes in interpreting cyclic variations in pulse pressure (delta PP). Severe ACP can result in significant delta PP, and yet no associated preload reserve. In this case, the variations in delta PP are linked to a cyclic effect on RV afterload (Figure 7, Film 21, Film 22). In this situation, study of SVC often avoids pointless or even dangerous refilling.
 Figure 7 : Patient on assisted ventilation because of ARDS, presenting severe ACP Figure 7 : Patient on assisted ventilation because of ARDS, presenting severe ACP
Blood volume expansion was unable to correct significant variations in pulse pressure |
REFERENCES
|
|
|
| |
|

