Click on each figure to increase it - If the enlarging does not function, check in your browser's option that 'javascript' is well activated.Definition Acute cor pulmonale (ACP) can be defined as a clinical situation in which the right ventricle (RV) is suddenly subjected to an excessive afterload. ACP is essentially seen during massive pulmonary embolism (PE), or in the setting of acute respiratory distress syndrome (ARDS). In these two situations, the right ventricular outflow impedance is suddenly increased, which reduces the ejection volume, producing right ventricular dilatation by augmentation of the end-systolic volume. Thus, ACP combines systolic and diastolic overload of the RV. This is taken into account in the echocardiographic definition, which combines paradoxical septal motion (linked to systolic overload) and right ventricular dilatation (linked to diastolic overload) (1). These two signs are always accompanied by abnormal left ventricular relaxation, revealed by the Doppler pattern of mitral flow (1).
Reminder : ventricular independanceA brief
reminder of this physiological phenomenon is necessary for a full
understanding of the echocardiographic anomalies observed in ACP. Normally,
the right and left ventricles contract at the same time, during
systole. When right ventricular ejection is hindered, as in ACP, right
ventricular contraction is prolonged, whereas contraction of the left
ventricle (LV) has already started its diastolic phase. The persistent
pressure of the RV then reverses the transseptal pressure gradient, the
pressure acting on the right ventricular face exceeding that on the
left ventricular face. This pushes the septum to the left, as seen in
ACP at the start of diastole (fig. 1). 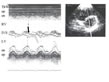 Figure 1: Paradoxical septal motion of acute cor pulmonale.
Guided by the two-dimensional image, a short-axis parasternal approach
is used for M-mode recording of the different structures crossed by the
ultrasound: ThW: anterior thoracic wall, ep: epicardium, en:
endocardium, RV: right ventricular chamber, IVS: interventricular
septum, LV: left ventricular chamber. The thin vertical arrows indicate
the end of ventricular contraction and the delayed end of right
ventricular contraction on the left, inducing protodiastolic septal
displacement, indicated by the thick black arrow. The septum remains
displaced throughout diastole, and is again pushed towards the right
ventricular chamber on the next left ventricular contraction (thick
white arrow). Theoretical normal septal movement is shown in dotted
lines to highlight the paradoxical motion. Figure 1: Paradoxical septal motion of acute cor pulmonale.
Guided by the two-dimensional image, a short-axis parasternal approach
is used for M-mode recording of the different structures crossed by the
ultrasound: ThW: anterior thoracic wall, ep: epicardium, en:
endocardium, RV: right ventricular chamber, IVS: interventricular
septum, LV: left ventricular chamber. The thin vertical arrows indicate
the end of ventricular contraction and the delayed end of right
ventricular contraction on the left, inducing protodiastolic septal
displacement, indicated by the thick black arrow. The septum remains
displaced throughout diastole, and is again pushed towards the right
ventricular chamber on the next left ventricular contraction (thick
white arrow). Theoretical normal septal movement is shown in dotted
lines to highlight the paradoxical motion. |
This
septal flattening persists throughout diastole, since the right
ventricular filling pressure is greater, because of diastolic overload
(fig. 1). But at the beginning of systole, the transseptal pressure
gradient is again reversed, and the septum is pushed towards the right
ventricular chamber (fig. 1). This results in the “paradoxical”
movement of the interventricular septum. Another important
physiological feature to take into account is the rigidity of the
pericardium surrounding the two ventricles. Any right ventricular
dilatation occurs at the expense of the left ventricle, which is
compressed (fig. 2). 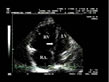 Figure 2: Major right ventricular dilatation during massive pulmonary embolism.
The right atrium (RA) and the right ventricle (RV) are very dilated on
this apical four-chamber view. The left ventricle (LV) appears
compressed by septal displacement (arrow). Figure 2: Major right ventricular dilatation during massive pulmonary embolism.
The right atrium (RA) and the right ventricle (RV) are very dilated on
this apical four-chamber view. The left ventricle (LV) appears
compressed by septal displacement (arrow). |
A
final physiological parameter to recall is that right ventricular size
varies with the quality of its filling: hypovolemia can markedly reduce
the dimensions of the right ventricular chamber, and this disorder must
be corrected before the echocardiographic examination if the results
are to be interpreted correctly. Insufficient venous return affecting
right ventricular size can be detected by ultrasound examination of the
vena cavae (2, 3, 4). In particular, in a ventilated patient, partial
or complete collapse of the superior vena cava on mechanical
insufflation indicates hypovolemia (FILM 1, FILM 2). Principal echocardiographic views used to study right ventricular function and detect ACPEchocardiographic
examination of the RV requires a long-axis view to measure chamber
size, and a small-axis view to evaluate the shape and movements of the
interventricular septum. To these should be added a view of the right
ventricular outflow tract used to assess the Doppler velocity and
morphology of pulmonary artery flow, and a Doppler recording of
tricuspid flow to detect tricuspid regurgitation and so measure the
transtricuspid gradient and deduce from it the pulmonary artery
systolicpressure (fig. 3).  Figure 3 : Four recordings of tricuspid regurgitant flow.
Doppler measurement of the peak velocity (V, arrow) is used to
calculate the transtricuspid pressure gradient, which is equal to 4V 2
. If we know the right atrial pressure, or the central venous pressure
(CVP), it can be added to this gradient to give the right ventricular
systolic pressure. If the exact CVP value is not known, it can be
estimated from the measurement of the diameter of the inferior vena
cava (diam) at the end of expiration ( CVP= 0.64diam + 0.77). Figure 3 : Four recordings of tricuspid regurgitant flow.
Doppler measurement of the peak velocity (V, arrow) is used to
calculate the transtricuspid pressure gradient, which is equal to 4V 2
. If we know the right atrial pressure, or the central venous pressure
(CVP), it can be added to this gradient to give the right ventricular
systolic pressure. If the exact CVP value is not known, it can be
estimated from the measurement of the diameter of the inferior vena
cava (diam) at the end of expiration ( CVP= 0.64diam + 0.77). |
Lastly, it is necessary to examine mitral flow velocity to identify any abnormal left ventricular relaxation (fig. 4). 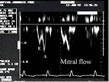 Figure 4 : Abnormal left ventricular relaxation.
Abnormal septal motion and compression of the left ventricular chamber
caused by right ventricular dilatation are seen in impaired left
ventricular filling, which is characteristic of abnormal relaxation:
the E-wave, of rapid filling, is reduced, whereas the A-wave linked to
atrial systole, is preeminent. Figure 4 : Abnormal left ventricular relaxation.
Abnormal septal motion and compression of the left ventricular chamber
caused by right ventricular dilatation are seen in impaired left
ventricular filling, which is characteristic of abnormal relaxation:
the E-wave, of rapid filling, is reduced, whereas the A-wave linked to
atrial systole, is preeminent. |
All
these effects and the respective views used to examine them are
detailed in the section on the principal echocardiographic views. When
a patient is breathing spontaneously, as is usually the case in
pulmonary embolism, the echocardiographic examination is done by the
transthoracic route (transthoracic echocardiography, or TTE). In a
patient on assisted ventilation, transesophageal echocardiography (TEE)
is easy and should therefore be used as it gives better images. This is
how ARDS patients are examined. Echocardiography is above all a
qualitative procedure. However, quantitative measurements can also be
made during or after acquisition of sequences. Table 1 gives the
principal normal values of our laboratory. Systolic overload detected by echocardiography As
explained above, systolic overload prolongs right ventricular systole,
while the ejection phase of this ventricle is often reduced (table 2).
This result in paradoxical septal motion linked to the specific
chronology of the pathological variations in transseptal pressure
gradient. This gradient (left ventricular pressure minus right
ventricular pressure) is always positive in physiological situations.
In ACP, it becomes negative at the end of systole/beginning of
diastole, remains negative during diastole because of right ventricular
diastolic overload, and again becomes positive at the start of systole.
These septal anomalies are clear in a small-axis view (Fig. 5, FILM 3, FILM 4) and can be analyzed in more detail in motion mode (M-mode) (fig. 1). 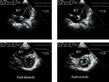 Figure 5 : Right ventricular deformation in acute cor pulmonale. In
a young female patient presenting acute cor pulmonale complicating
acute respiratory distress syndrome, the apical four-chamber view
(above) shows right ventricular dilatation accompanied by a rounded
appearance of the apex, at the end of both diastole and systole. The
short-axis parasternal view (below) shows the loss of this crescent
appearance of the right ventricular chamber, which becomes oval-shaped.
The outcome is that the right ventricle resembles the left ventricle: a
180° rotation of the probe could lead to confusion in the long axis, as
the right ventricle then appears on the right of the image, in the
place of the left ventricle. In some countries (Russia, for example),
the recording is presented backwards and so this confusion is possible.
To avoid confusion remember that the tricuspid valve plane is always
situated above the mitral valve plane. Figure 5 : Right ventricular deformation in acute cor pulmonale. In
a young female patient presenting acute cor pulmonale complicating
acute respiratory distress syndrome, the apical four-chamber view
(above) shows right ventricular dilatation accompanied by a rounded
appearance of the apex, at the end of both diastole and systole. The
short-axis parasternal view (below) shows the loss of this crescent
appearance of the right ventricular chamber, which becomes oval-shaped.
The outcome is that the right ventricle resembles the left ventricle: a
180° rotation of the probe could lead to confusion in the long axis, as
the right ventricle then appears on the right of the image, in the
place of the left ventricle. In some countries (Russia, for example),
the recording is presented backwards and so this confusion is possible.
To avoid confusion remember that the tricuspid valve plane is always
situated above the mitral valve plane. |
A
systolic overload that persists for more than a few hours also results
in morphological changes in the right ventricular chamber: First,
the shape of the right ventricle changes. On the long axis, the apical
region, which is normally triangular, becomes rounded. On the short
axis, the right ventricle changes from a crescent to an oval shape.
Together with dilatation, this deformation has the effect that the
shape of the right ventricular chamber, which is normally very
different from that of the left ventricular chamber, comes to resemble
it somewhat (fig. 5). Second, incipient hypertrophy of
the free wall of the RV occurs, with accentuation of muscular
trabeculae (fig. 6 and 7) and wall thickening (figs. 7 and 8). Values
around 0.6 cm are common for the right ventricular free wall whose
thickness normally does not exceed 0.3 cm. But parietal hypertrophy is
never as marked as that seen in chronic cor pulmonale where values of
about 1 cm are common. 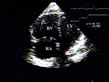 Figure 6 : Rapid right ventricular hypertrophy in acute cor pulmonale, first example. In
this patient presenting acute cor pulmonale complicating acute
respiratory distress syndrome, hypertrophic trabeculae are seen in the
dilated right ventricular chamber (arrow). Figure 6 : Rapid right ventricular hypertrophy in acute cor pulmonale, first example. In
this patient presenting acute cor pulmonale complicating acute
respiratory distress syndrome, hypertrophic trabeculae are seen in the
dilated right ventricular chamber (arrow). |
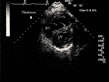 Figure 7: Rapid right ventricular hypertrophy in acute cor pulmonale, second example. In
this female patient presenting acute cor pulmonale complicating massive
pulmonary embolism, hypertrophic trabeculae are seen in the dilated
right ventricular chamber (arrows). Note also the thickness of the
wall: 0.7 cm. Figure 7: Rapid right ventricular hypertrophy in acute cor pulmonale, second example. In
this female patient presenting acute cor pulmonale complicating massive
pulmonary embolism, hypertrophic trabeculae are seen in the dilated
right ventricular chamber (arrows). Note also the thickness of the
wall: 0.7 cm. |
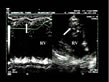 Figure 8: Rapid right ventricular hypertrophy in acute cor pulmonale, third example.
In this patient presenting acute cor pulmonale complicating acute
respiratory distress syndrome, hypertrophy of the right ventricular
wall is clearly visible on a transgastric approach in M-mode (on left,
arrow). Figure 8: Rapid right ventricular hypertrophy in acute cor pulmonale, third example.
In this patient presenting acute cor pulmonale complicating acute
respiratory distress syndrome, hypertrophy of the right ventricular
wall is clearly visible on a transgastric approach in M-mode (on left,
arrow). |
Severe systolic overload
leads to a reduced ejection volume, which can be evaluated by the
Doppler time-velocity integral of the pulmonary flow (table 2). A
biphasic appearance indicates a large increase in resistance to
pulmonary blood flow (fig. 9, FILM 5, FILM 6).
The reduction in ejection volume is compensated for a while by
tachycardia, but in the end leads to a drop in cardiac flow. The onset
of ACP can therefore precipitate acute circulatory insufficiency. Table 2 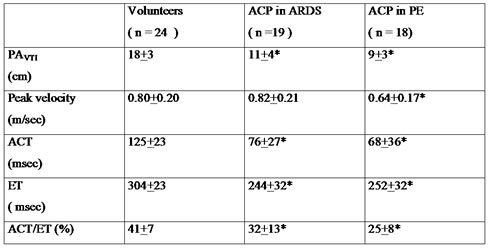
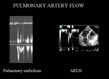 Figure 9: Biphasic appearance of pulmonary artery flow.
The sudden rise in resistance to pulmonary blood flow alters the
Doppler profile of pulmonary artery flow, which becomes biphasic. This
anomaly is also seen in chronic pulmonary arterial hypertension. Figure 9: Biphasic appearance of pulmonary artery flow.
The sudden rise in resistance to pulmonary blood flow alters the
Doppler profile of pulmonary artery flow, which becomes biphasic. This
anomaly is also seen in chronic pulmonary arterial hypertension. |
Long-axis
measurement of the right ventricular diastolic and systolic areas can
be used to calculate the fractional reduction in right ventricular
area. But this measurement, which is very useful when studying the
quality of left ventricular systolic function, is, in our experience,
of no value when studying the RV. This is because there is no fixed
normal physiological value, and because pathological variations in this
parameter can occur for a while in the same direction as variations in
afterload. Diastolic overload detected by echocardiography Diastolic
overload of the right ventricle dilates this chamber at the end of
diastole. This dilatation is easy to observe but difficult to measure
accurately. The particular shape of the right ventricle means that any
volume calculation by echocardiography is impossible in practice.
However, long-axis measurement of the right ventricular area is
straightforward using an apical four-chamber view or the
transesophageal route. It is therefore possible to establish the ratio
of the end-diastolic areas of the two ventricles, which is normally
less than or equal to 0.6. A ratio above 0.6 can therefore be
considered as indicative of right ventricular dilatation. But this is
not necessarily pathological and must be interpreted in the light of
other echocardiographic signs, notably the presence or absence of a
septal anomaly suggesting systolic overload. Likewise, a normal Doppler
echocardiogram of mitral flow, when the RV seems slightly dilated,
rules out a pathological cause. On the other hand, a E/A ratio equal to
or lower than one indicates marked right ventricular dilatation and is
always pathological (fig. 10, FILM 7, FILM 8). 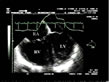 Figure 10: Severe acute cor pulmonale in acute respiratory distress syndrome.
This long-axis view by the transesophageal approach shows that the
right ventricular (RV) area exceeds the left ventricular (LV) area. The
right atrium (RA) is also very dilated. Figure 10: Severe acute cor pulmonale in acute respiratory distress syndrome.
This long-axis view by the transesophageal approach shows that the
right ventricular (RV) area exceeds the left ventricular (LV) area. The
right atrium (RA) is also very dilated. |
The
right ventricular dilatation observed during acute cor pulmonale is
associated with right atrial dilatation (fig. 2, fig. 10), and
enlargement of the inferior vena cava (fig. 11). 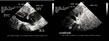 Figure 11: Dilatation of the inferior vena cava.
A: in this female patient presenting acute right ventricular
insufficiency, the inferior vena cava (IVC) and a hepatic vein (HV) are
dilated. B: after injection of contrast agent in a vein of the superior
caval network, the agent flows back into the IVC and IHV, indicating
tricuspid regurgitation. Figure 11: Dilatation of the inferior vena cava.
A: in this female patient presenting acute right ventricular
insufficiency, the inferior vena cava (IVC) and a hepatic vein (HV) are
dilated. B: after injection of contrast agent in a vein of the superior
caval network, the agent flows back into the IVC and IHV, indicating
tricuspid regurgitation. |
There is
also tricuspid regurgitation which can be seen by contrast ultrasound
(fig. 11), and which is utilized to measure pulmonary artery systolic
pressure, using Doppler echocardiography (fig. 3). When right atrial
pressure exceeds left atrial pressure, the foramen ovale, which had
remained permeable, may reopen. This anomaly, which induces a
right-left shunt, can be detected by contrast ultrasound (fig. 12) or
color Doppler (fig. 13, FILM 9) (5, 6). It causes paradoxical arterial embolism in thromboembolic disease (7). 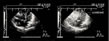 Figure 12: Contrast echocardiographic detection of patent foramen ovale (PFO).
In this patient massive pulmonary embolism is complicated by acute cor
pulmonale which is indicated by dilatation of the right atrium (RA) and
right ventricle (RV), with reduced left ventricular (LV) size on an
apical four-chamber view (A). Injection of contrast agent in a
peripheral vein (B) opacifies the right chambers, but the contrast
agent quickly passes into the left chambers, indicating patency of the
foramen ovale. Figure 12: Contrast echocardiographic detection of patent foramen ovale (PFO).
In this patient massive pulmonary embolism is complicated by acute cor
pulmonale which is indicated by dilatation of the right atrium (RA) and
right ventricle (RV), with reduced left ventricular (LV) size on an
apical four-chamber view (A). Injection of contrast agent in a
peripheral vein (B) opacifies the right chambers, but the contrast
agent quickly passes into the left chambers, indicating patency of the
foramen ovale. |
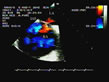 Figure 13:Color Doppler detection of patent foramen ovale (PFO).
In this patient presenting acute cor pulmonale complicating massive
pulmonary embolism, color Doppler examination of the interatrial septum
(IAS) shows turbulent flow towards the probe (red), across the IAS and
into the left atrium (LA). Note also the marked dilatation of the right
atrium (RA). Figure 13:Color Doppler detection of patent foramen ovale (PFO).
In this patient presenting acute cor pulmonale complicating massive
pulmonary embolism, color Doppler examination of the interatrial septum
(IAS) shows turbulent flow towards the probe (red), across the IAS and
into the left atrium (LA). Note also the marked dilatation of the right
atrium (RA). |
Effects of acute cor pulmonale on the left ventricle Sudden
right ventricular dilatation within in an inextensible pericardium
results in left ventricular compression, which is easily seen on
echocardiographic examination (fig. 2, 5, 6, 7 and 10). Acute cor
pulmonale therefore reduces left ventricular diastolic dimensions
(tables 2 and 3) (8, 9, 10). In massive pulmonary embolism, this sudden
drop in preload causes acute circulatory insufficiency (FILM 7).
In ARDS, the decrease in left ventricular preload is usually more
progressive, but may also contribute to circulatory insufficiency (FILM 10, FILM 11, FILM 12). Left
ventricular compression by right ventricular dilatation contributes
more to the reduction in LV diastolic filling if it occurs when the
pulmonary circulation is partly obstructed: proximal obstruction by a
thrombus in massive PE, distal obstruction by the action of high
alveolar pressure on pulmonary capillaries in ARDS managed by assisted
ventilation (11). In addition to the reduction in left
ventricular diastolic dimensions, Doppler echocardiography reveals
abnormal relaxation which is seen in predominance of the A-wave over
mitral flow (table 2 and 3) (fig. 4, FILM 13, FILM 14). Table 3
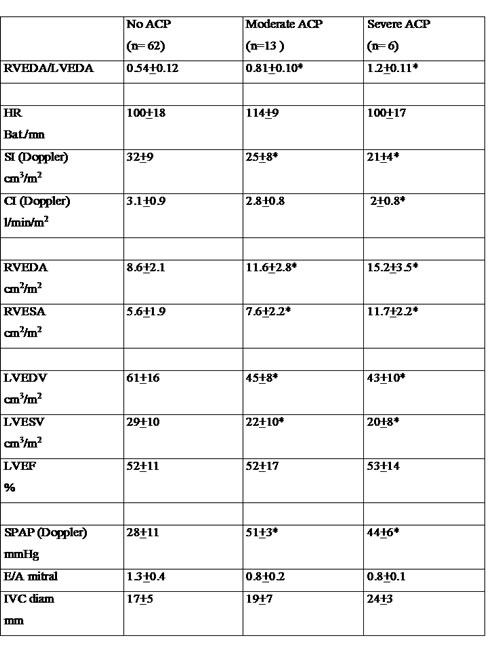 Table 4 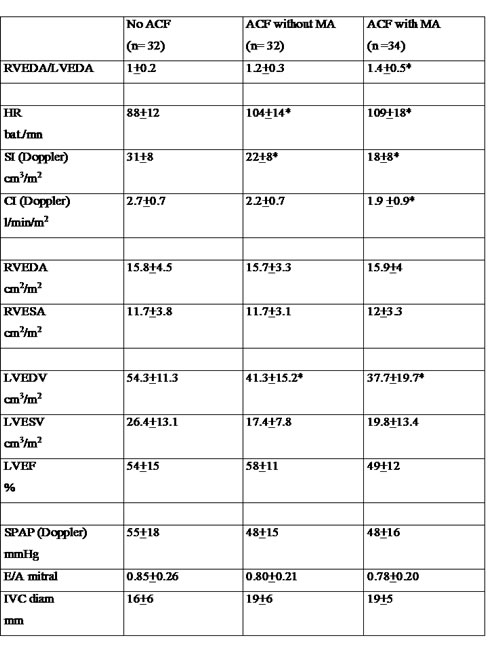 Acute Cor Pulmonale complicating massive pulmonary embolismThe
most frequent cause of acute cor pulmonale is obstruction of at least
two lobar arteries by massive PE. Kasper was the first to underscore
the value of echocardiography when assessing a patient suspected to
have PE (12). He quantified right ventricular dilatation using the
ratio of the ventricular diameters in M-mode. In view of the
deformation of the right ventricle when it dilates, the area ratio we
have proposed (1) is more reliable: a simple ratio of diameters ignores
apical deformation. Using our echocardiographic definition of
ACP, which combines right ventricular dilatation and paradoxical septal
motion, we noted ACP in 61% of 161 successive patients presenting
massive PE. Diagnosis is usually made by TTE, since assisted
ventilation is rarely used in a patient presenting massive PE. However,
in certain emergency patients on assisted ventilation because of
cardiorespiratory arrest, TEE can give an immediate diagnosis by
detecting ACP, and even by visualizing the thrombus (13) (fig. 14, FILM 15, FILM 16, FILM 17, FILM 18). A thrombus in the right chamber is rarely visualized by TTE (FILM 19). 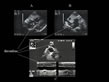 Figure 14: Visualization of a thrombus in the right chamber and pulmonary artery.
Transthoracic echocardiography reveals a thrombus floating in the
atrium right (A, B). In another patient (C), a floating thrombus is
visualized in the right pulmonary artery. Figure 14: Visualization of a thrombus in the right chamber and pulmonary artery.
Transthoracic echocardiography reveals a thrombus floating in the
atrium right (A, B). In another patient (C), a floating thrombus is
visualized in the right pulmonary artery. |
Acute
Cor Pulmonale during PE indicates a major obstruction but is not always
accompanied by circulatory insufficiency, as defined by the need to use
vasoactive drugs to maintain systolic blood pressure above 90 mmHg
(10). Circulatory insufficiency is, however, frequent: we observed it
in two thirds of patients with ACP complicating PE (10). When the
patient does not develop metabolic acidosis, the prognosis of this
circulatory insufficiency is excellent, with vasoactive support for a
few hours using dobutamine in first-line treatment (14), and then
adrenaline or norepinephrine if dobutamine does not rapidly maintain
the blood pressure. Metabolic acidosis marked by a base deficit above 5
mmol/l is a serious sign, and the only sign in our opinion that
justifies use of thrombolytic agents (10). Acute Cor Pulmonale complicating acute respiratory distress syndrome In
this setting, two associated factors combine to raise right ventricular
outflow impedance: 1/ underlying pulmonary disease, which usually
causes permanent diffuse arteriolar obstructions (15); 2/ assisted
ventilation (16), which results in microvascular, intermittent or
permanent obstructions, by elevation of transpulmonary pressure (17,
18). Bedside echocardiography provided the first description of
this complication of ARDS, at a time when high tidal volumes (13 ml/kg)
were used (FILM 20, FILM 21
) (19). The frequency of this complication was 61% then, a value close
to mortality of the syndrome. It is now known that these tidal volumes,
and the high plateau pressure they induce, are excessive. Reduction in
plateau pressure to below 30 cm H 2O significantly reduces the
frequency of ACP to about 25% (9) (FILM 22, FILM 23). The
onset of ACP during ARDS is generally more gradual than during PE and
is observed after a certain time on assisted ventilation (9). In
certain patients ACP may occur on introduction of assisted ventilation (FILM 24, FILM 25 ) or can be triggered by untimely adjustment of respirator settings (FILM 26, FILM 27, FILM 28
). In some patients, the later onset of ACP indicates a
fibroproliferative phase, which can be arrested by corticosteroid
therapy (FILM 29, FILM 30). If ACP occurs during ARDS, the following measures should be implemented immediately: - reduce plateau pressure to below 25 cm H 2O
- lower PEEP to below 8 cm H 2O
- reduce
PaCO 2 to below 60-65 mmHg by use of a heater/humidifier in place of
the filter (20), possibly by increasing respiratory frequency in
certain patients. However, this maneuver is rarely effective and by
generating an intrinsic PEEP often raises the plateau pressure, at the
expense of right ventricular ejection (21). Remember that hypercapnia,
which leads to systemic vasodilatation, has the reverse effect on the
pulmonary circulation, resulting in arteriolar vasoconstriction (22).
- prone positioning if the ratio PaO 2/FIO 2 remains below 100 mmHg
- use TEE to check the absence of proximal PE
If
ACP is accompanied by insufficiency circulatory, the most suitable
vasoactive drug is norepinephrine, which restores systemic blood
pressure and so improves right coronary flow and right ventricular
systolic function (FILM 25, FILM 31). When
ACP appears after more than one week of assisted ventilation in a
patient whose lung compliance is deteriorating, and in whom hypercapnia
is increasing, this combination is strongly suggestive of a
fibroproliferative phase. We then always use corticosteroid therapy. Lastly, inhaled NO can also afford rapid relief and reduce or eliminate signs of ACP (FILM 32, FILM 33). In
our experience, immediate implementation of these measures, which
presupposes rapid echocardiographic diagnosis, has meant that ACP no
longer results in excess mortality in ARDS. ACP can greatly reduce the
likelihood of cure if specific and timely measures are not taken (19). Acute Cor Pulmonale in other clinical settings Sudden
obstruction of the pulmonary circulation by a gas or fat embolism
causes acute pulmonary artery hypertension, which is often rapidly
reversible. We have reported a case of ACP triggered by intravenous
injection of drug powder (FILM 34) (23). Acidosis,
whether respiratory or metabolic, induces pulmonary artery
hypertension, which has long been known to complicate primary lactic
acidosis (24). We have observed several cases of ACP complicating
primary lactic acidosis (1). Lactic acidosis caused by septic shock may also be involved in the onset of ACP (FILM 35, FILM 36). References 1 Jardin F, Dubourg O, Bourdarias JP: Echocardiographic pattern of acute cor pulmonale. Chest 1997;111:209-217 2
Barbier Ch, Loubières Y, Schmit Ch, Hayon J, Ricome JL, Jardin F,
Vieillard-Baron A: Respiratory changes in inferior vena cava diameter
are helpful in predicting fluid responsiveness in ventilated septic
patients. Intensive Care Med 2004;30:1740-1746 3
Vieillard-Baron A, Augarde R, Prin S, Page B, MD, Beauchet A, Jardin
F:Influence of superior vena caval zone conditions on cyclic changes in
right ventricular outflow during respiratory support. Anesthesiology
2001;95:1083-1088 4 Vieillard-Baron A, Chergui K, Rabiller A,
Peyrouset O, Page B, Beauchet A, Jardin F: Superior vena cava
collapsibility as a gauge of volume status in ventilated septic
patients. Intensive Care Med 2004;30:1734-1739 5 Dubourg O,
Bourdarias JP, Farcot JC, Guéret P, Terjman M, Ferrier A, Rigaud M,
Bardet J: Contrast echocardiographic visualization of cough-induced
right to left shunt through a patent foramen ovale. J Amer Col Cardiol
1984;4:587-594 6 Konstadt S, Louie E, Black S, Rao T, Scanlon
P: Intraoperative detection of patent foramen ovale by transesophageal
echocardiography. Anesthesiology 1991; 74:212-216 7 Lechat Ph,
Mas JL, Lascault G, Loron PH, Theard M, Klimczac M, Drobinsky G, Thomas
D, Grosgogeat Y: Prevalence of patent foramen ovale in patients with
stroke. N Engl J Med 1988;318:1149-1152 8 Jardin F, Dubourg O,
Guéret P, Delorme G, Bourdarias JP: Quantitative two-dimensional
echocardiograohy in massive pulmonary embolism: emphasis on ventricular
interdependence and leftward septal displacement. JACC
1987;10:1201-1206 9 Vieillard-Baron A, Schmitt JM, Augarde R,
Fellahi JL, Prin, Page B, Beauchet A, Jardin F: Acute cor pulmonale in
ARDS submitted to protective ventilation: incidence, clinical
implications and prognosis. Crit Care Med 2001;29:1551-1555 10
Vieillard-Baron A, Page B, MD, Augarde R, Prin S, Qanadli S, MD,
Beauchet A, Dubourg O, Jardin F: Acute cor pulmonale in massive
pulmonary embolism: incidence, echocardiographic pattern, clinical
implications and recovery rate. Intensive Care Med 2001;27:1481-1486 11
Jardin F, Vieillard-Baron A: Right ventricular function and positive
pressure ventilation in clinical practice: from hemodynamic subsets to
respirator setting. Intensive Care Med 2003;29:1426-1434 12
Kasper W, Meinertz T, Kerstin F, Löllgren H, Limbourg P, Just H
Echocardiography in assessing acute pulmonary hypertension due to
pulmonary embolism. Am J Cardiol 1980;45:567-572 13
Vieillard-Baron A, Quanadli S, Antakly Y, Fourme T, Loubières Y, Jardin
F, Dubourg O: Transesophageal echocardiography for the diagnosis of
pulmonary embolism with acute cor pulmonale: a comparison with
radiologic procedures. Intensive Care Med 1998; 24:429-433 14
Jardin F, Genevray B, Brun-Ney D, Margairaz A: Dobutamine: a
hemodynamic evaluation in pulmonary embolism shock. Crit Care Med
1985;13:1909-1012 15 Zapol W, Jones R: Vascular component of
ARDS: clinical pulmonary hemodynamics and morphology. Am Rev Respir Dis
1987;136:471-474 16 Jardin F, Delorme G, Hardy A, Auvert B,
Beauchet A, Bourdarias JP: Reevaluation of hemodynamic consequences of
positive pressure ventilation: emphasis on cyclic right ventricular
afterloading by mechanical lung inflation. Anesthesiology
1990,72:966-970 17 Vieillard-Baron A, Loubières Y, Schmitt JM,
Page B, Dubourg O, Jardin F: Cyclic changes in right ventricular
outflow impedance during mechanical ventilation. J Appl Physiol
1999;87:1644-1650 18 Schmitt JM, Vieillard-Baron A, Augarde R,
Prin S, Page B, Jardin F: PEEP titration in ARDS patients: impact on
right ventricular outflow impedance evaluated by pulmonary artery
Doppler flow velocity measurements. Crit Care Med, in press. 19
Jardin F, Gueret P, Dubourg O, Farcot JC, Margairaz A, Bourdarias JP:
Two-dimensional echocardiographic evaluation of right ventricular size
and contractility in acute respiratory failure. Crit Care Med
1985;13:952-956 20 Prin S, Chergui K, Augarde R, Page B, Jardin
F, Vieillard-Baron A: Ability and safety of a heated humidifier to
control hypercapnic acidosis in severe ARDS. Intensive Care Med
2002;28:1756-1760 21 Vieillard-Baron A, Prin S, Augarde R,
Desfonds P, Page B, Beauchet A, MD, Jardin F: Increasing respiratory
rate to improve CO 2 clearance during mechanical ventilation is not a
panacea in acute respiratory failure. Crit Care Med, 2002, 30:
30:1407-1412 22 Balanos G, Talbot N, Dorrington K, Robins P:
Human pulmonary vascular response to 4h of hypercapnia and hypocapnia
measured using Doppler echocardiography. J Appl Physiol
2003;94:1543-1551 23 Jullien T, Valtier B, Vieillard-Baron A,
Bourdarias JP, Jardin F: Rapidly reversible acute cor pulmonale after
intravenous injection of crushed dextromoramide (Palfium) pills.
Intensive Care Med 1996;270-271 24 Latif M, Weil M: Circulatory deficit during phenformin lactic acidosis. Intensive Care Med 1979;5:135-139 Videos Index >>>
|

