| |
|
|
Cardiac tamponade |
|
|
|
Sunday, 17 October 2004 |
Click on each figure to increase it - If the enlarging does not function, check in your browser's option that 'javascript' is well activated.The term cardiac tamponade (CT) designates the circulatory consequences of compression caused by accumulation of blood or fluid in the pericardial space. The poor distensibility of the normal pericardium means that rapid pericardial effusion results in compression and hinders filling of the cardiac chambers. This explains the hemodynamic effect of acute circulatory failure, with a low arterial pressure and a high central venous pressure (values above 20 cm H 2 O are common).
Echography has simplified the diagnostic approach to CT, which is a circulatory emergency. Diagnosis previously involved a perilous procedure: exploratory puncture of the pericardium. Echocardiographic exploration also detects signs of incipient compression before it has a major impact. Observation of these signs has refined the pathophysiological analysis of CT. Echography has simplified the diagnostic approach to CT, which is a circulatory emergency. Diagnosis previously involved a perilous procedure: exploratory puncture of the pericardium. Echocardiographic exploration also detects signs of incipient compression before it has a major impact. Observation of these signs has refined the pathophysiological analysis of CT. PATHOPHYSIOLOGICAL ASPECTSThe mechanism of CT is complex and we need to consider several aspects in order to explain the phenomena observed by invasive or echographic investigation. 1- Atrioventricular competition within the pericardium (Fig. 1): Provided they remain within their physiological limits, distension of the atria and ventricles is not limited by a healthy pericardial space. The sum of the chamber volumes is greater in diastole than in systole. When a pathological effusion occurs, atrioventricular competition is such that the sum of the chamber volumes is constant throughout the cardiac cycle. When the compression is greater, this constant is smaller. In a physiological setting, sudden reduction in ventricular dimensions at the start of systole creates pericardial depression which drives venous return, and the atria fill by anterograde systolic acceleration of the vena caval flow. The ventricle dilates on diastole and the ventricular depression thus created aspirates the blood of the atrium towards the ventricle (E-wave of rapid filling), but also from the vena cavae towards the atrium, inducing anterograde diastolic acceleration of caval flow. At end-diastole, atrial systole completes ventricular filling (A-wave).  Fig. 1 : In the normal physiological situation (upper part of the figure, V: ventricle, A: atrium), the reduction of ventricular volume on systole (S) lowers the pericardial pressure and facilitates atrial filling by anterograde accentuation of caval flow (systolic filling wave, see Fig. 18). In protodiastole (PD), ventricular dilatation raises the pericardial pressure, but the aspiration effect created by this dilatation is greater and favors rapid filling (E) (On the recording of the atrial pressure curve, this aspiration effect is responsible for the hollow y.) At the same time, the atrium is filled by new anterograde acceleration of caval flow (diastolic filling wave, see Fig. 18). In end-diastole (TD), ventricular filling is completed by atrial systole (SA). During cardiac tamponade (lower part of the figure), the sudden rise in pericardial pressure in protodiastole compresses the atrium and reverses the caval flow (note that the hollow y is missing on a recording of atrial pressure, and that on a recording of caval flow there is no diastolic wave, see Fig. 18). In end-diastole, only atrial systole participates in ventricular filling. The catastrophic consequences of atrial fibrillation in this situation are easy to imagine. . |
In the pathological setting of CT (Fig. 2, Film 1), the atrium can only be filled if the ventricle empties, and vice versa. On systole, the pericardial depression created by the reduction in ventricular dimensions enables atrial filling, and the anterograde systolic acceleration of caval flow is preserved. From the start of diastole, the increase in ventricular volume raises pericardial pressure and atrial compression. The atrium empties towards the zone of least resistance, extracardiac and retrograde: the E-wave of rapid filling is greatly reduced and there is a retrograde diastolic acceleration of caval flow and diastolic atrial collapse (Film 2). At end-diastole, active atrial contraction supplies ventricular filling: the A-wave becomes predominant.
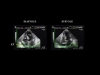 Fig. 2: An apical four-chamber view can be used to observe the pushing in of the atrial wall by the pericardial effusion (EP) in diastole, and the re-expansion of the right atrium (OD) in systole. VD: right ventricle, VG: left ventricle. Fig. 2: An apical four-chamber view can be used to observe the pushing in of the atrial wall by the pericardial effusion (EP) in diastole, and the re-expansion of the right atrium (OD) in systole. VD: right ventricle, VG: left ventricle.
|
2- Ventricular-ventricular competition within the pericardium (Fig. 3): Pericardial effusion limits the volume shared by the ventricles. The external pressure it exerts more readily affects the right ventricle whose muscle wall is less resistant. At the start of diastole, the sudden rise in pericardial pressure caused by the increase in ventricular volume presses against the free wall of the right ventricle, which may even cave in (Fig 4, Film 3).
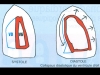 Fig. 3: The sudden rise in pericardial pressure during diastole preferentially compresses the right ventricle (VD) whose wall is easier to depress than that of the left ventricle (VG). The result is diastolic collapse of the right ventricle. |
 Fig. 4: On this motion mode recording, note how pericardial effusion (EP) pushes back (arrow) the free wall of the right ventricle (VD) leading to diastolic reduction of the right ventricular chamber. |
Impact on pericardial pressure of respiratory variations in pleural pressure (Fig. 5, Film 3) :Variations in pleural pressure are fully transmitted to the pericardial chamber. The negative inspiratory pressure accordingly reduces the external pressure on the chambers. Positive pressure would increase the external pressure. Fig. 5 : The external pressure exerted on the ventricular chamber is the sum of the pleural (negative) and pericardial (positive, produced by the elastic recoil of the pericardium) pressures. This external pressure is diminished by inspiration, which lowers the pleural pressure. Fig. 5 : The external pressure exerted on the ventricular chamber is the sum of the pleural (negative) and pericardial (positive, produced by the elastic recoil of the pericardium) pressures. This external pressure is diminished by inspiration, which lowers the pleural pressure. |
4 - Ventricular interaction through the septum (Fig. 6):This is another aspect of the ventricular-ventricular competition caused by the restriction of the pericardial space. Inspiration, which steepens the gradient favorable to venous return fed by extrathoracic blood vessels, encourages expansion of the right ventricle. As this expansion cannot occur through encroachment on the pericardial space, it happens through septal shift towards the left ventricular chamber (Figure 7, Film 4, Film 5). The dimensions and distensibility of the left ventricular chamber are thus reduced, which blocks pulmonary venous return. Expiration on the other hand cancels the gradient favorable to venous return, which is therefore interrupted. The resulting reduction in size of the right ventricle allows the septum to return to its place, and the pulmonary venous return is unblocked, permitting filling of the left ventricle
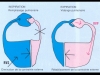 Fig. 6: On inspiration, pleural depression favors systemic venous return (RVS) and right ventricular filling. Because of a lack of room in the pericardial space, increase in right ventricular diastolic volume shifts the septum leftwards and reduces the diastolic dimensions of the left ventricle. The venous pulmonary pressure increases, and the pulmonary venous return (RVP) is blocked, which leads to blood storage in the pulmonary circulation. On expiration, the systemic venous return is stopped, the septum returns to its place, the pulmonary venous return is restored, and the pulmonary venous circulation empties into the left ventricle. Fig. 6: On inspiration, pleural depression favors systemic venous return (RVS) and right ventricular filling. Because of a lack of room in the pericardial space, increase in right ventricular diastolic volume shifts the septum leftwards and reduces the diastolic dimensions of the left ventricle. The venous pulmonary pressure increases, and the pulmonary venous return (RVP) is blocked, which leads to blood storage in the pulmonary circulation. On expiration, the systemic venous return is stopped, the septum returns to its place, the pulmonary venous return is restored, and the pulmonary venous circulation empties into the left ventricle. |
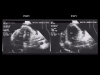 Fig. 7: On this apical four-chamber view, note the variations in size of the right (VD) and left (VG) ventricles on inspiration and expiration. |
5 - Mechanical intervention of the pulmonary vessels ("pooling") :This is the direct consequence of what has happened before. On inspiration, the right ventricle is supplied with blood and produces a substantial ejection which is stored in the pulmonary circulation, which cannot be emptied because it is blocked by the downstream obstacle constituted by a rigid left ventricle. Expiratory improvement in left ventricular distensibility lifts the blockage of the pulmonary circulation, and the elastic recoil of the pulmonary circulation drives the blood towards the left chambers on expiration. HEMODYNAMIC ASPECTS Cardiac output plummets during CT, and heart rate accelerates. The arteriovenous difference increases. This precarious hemodynamic situation is accompanied by metabolic acidosis. Systemicarterial pressure is low and this drop in pressure is particularly marked on inspiration. Monitoring of arterial pressure reveals an inspiratory reduction in systemic pulse (1,2), which may even be abolished (pulsus paradoxus) (Fig. 8).
 Fig. 8: In a patient presenting a clinical picture of cardiac tamponade, simultaneous recording of an electrocardiogram (ECG), radial artery pressure (AR), pulmonary artery pressure (AP), pulmonary capillary pressure (or pulmonary artery occlusion pressure, CP), right atrial pressure (OD), and esophageal pressure (Pl), which reflects pleural pressure (Pl). On inspiration, marked by a drop in pleural pressure, radial pressure decreases, possibly leading even to complete disappearance of the pulse (arrows). With the pulmonary pulse note also the phase contrast, which is accentuated on inspiration. Lastly, note the equalization of the right (OD) and left (CP) filling pressures in the inspiratory phase, and the restoration of a physiological gradient (CP>OD) in the expiratory phase. Fig. 8: In a patient presenting a clinical picture of cardiac tamponade, simultaneous recording of an electrocardiogram (ECG), radial artery pressure (AR), pulmonary artery pressure (AP), pulmonary capillary pressure (or pulmonary artery occlusion pressure, CP), right atrial pressure (OD), and esophageal pressure (Pl), which reflects pleural pressure (Pl). On inspiration, marked by a drop in pleural pressure, radial pressure decreases, possibly leading even to complete disappearance of the pulse (arrows). With the pulmonary pulse note also the phase contrast, which is accentuated on inspiration. Lastly, note the equalization of the right (OD) and left (CP) filling pressures in the inspiratory phase, and the restoration of a physiological gradient (CP>OD) in the expiratory phase.
|
Pulmonaryartery pressure also is subject to large respiratory variations which are almost completely out of phase with the systemic variations. The pulmonary pulse is amplified by inspiration and diminished by expiration (Fig. 8, Fig. 9) (2).
 Fig. 9: In a patient presenting a clinical picture of cardiac tamponade, three successive recordings of the electrocardiogram (ECG), radial artery pressure (AR), pulmonary artery pressure (AP), pulmonary capillary pressure (or pulmonary artery occlusion pressure, CP), right atrial pressure (OD), and esophageal pressure (Oe), which reflects pleural pressure (in: inspiration, exp: expiration). These three recordings were made during progressive removal of fluid effusion by pericardial puncture: left, baseline recording before fluid removal; middle, recording after removal of 250 cc; right, recording after removal of 500 cc. Note the progressive restoration of a correct radial artery pressure, with reappearance of a pulse in the inspiratory phase. Note too the phase contrast between the systemic pulse and the pulmonary pulse. Fig. 9: In a patient presenting a clinical picture of cardiac tamponade, three successive recordings of the electrocardiogram (ECG), radial artery pressure (AR), pulmonary artery pressure (AP), pulmonary capillary pressure (or pulmonary artery occlusion pressure, CP), right atrial pressure (OD), and esophageal pressure (Oe), which reflects pleural pressure (in: inspiration, exp: expiration). These three recordings were made during progressive removal of fluid effusion by pericardial puncture: left, baseline recording before fluid removal; middle, recording after removal of 250 cc; right, recording after removal of 500 cc. Note the progressive restoration of a correct radial artery pressure, with reappearance of a pulse in the inspiratory phase. Note too the phase contrast between the systemic pulse and the pulmonary pulse. |
Left atrial pressure, determined by measurement of the pulmonary artery occlusion pressure, is abnormally high (3) with a mean value close to the pericardial pressure. Transmural pressure is therefore very low but varies with respiration: nil on inspiration but slightly positive on expiration (Fig. 10) (4,5).  Fig. 10: In a patient presenting a clinical picture of cardiac tamponade, three successive recordings of the electrocardiogram (ECG), pulmonary artery pressure (AP), pulmonary capillary pressure (or pulmonary artery occlusion pressure, CP), right atrial pressure (OD), pericardial pressure obtained on puncture (P), and pleural pressure (Pl), obtained on simultaneous removal of pleural effusion. These three recordings were made during progressive removal of the fluid effusion by pericardial puncture: left, baseline recording before removal; middle, recording after removal of 250 cc; right, recording after removal of 500 cc. Note the high (17 mmHg) right atrial pressure (or central venous pressure) which is equal to the pericardial pressure, on the left of the recording. The removal of the pericardial effusion results in a drop in right atrial pressure, accompanied by a much more marked decrease in pericardial pressure, which finally becomes equal to the pleural pressure at the end of fluid removal. The transmural central venous pressure, which was initially nil, therefore becomes positive. Fig. 10: In a patient presenting a clinical picture of cardiac tamponade, three successive recordings of the electrocardiogram (ECG), pulmonary artery pressure (AP), pulmonary capillary pressure (or pulmonary artery occlusion pressure, CP), right atrial pressure (OD), pericardial pressure obtained on puncture (P), and pleural pressure (Pl), obtained on simultaneous removal of pleural effusion. These three recordings were made during progressive removal of the fluid effusion by pericardial puncture: left, baseline recording before removal; middle, recording after removal of 250 cc; right, recording after removal of 500 cc. Note the high (17 mmHg) right atrial pressure (or central venous pressure) which is equal to the pericardial pressure, on the left of the recording. The removal of the pericardial effusion results in a drop in right atrial pressure, accompanied by a much more marked decrease in pericardial pressure, which finally becomes equal to the pleural pressure at the end of fluid removal. The transmural central venous pressure, which was initially nil, therefore becomes positive.
|
Centralvenous pressure is abnormally high with a mean value identical to that of the pericardial pressure (equalization of pressures). The transmural right atrial pressure is therefore permanently close to zero. It undergoes few respiratory variations. Transmural pressure is slightly positive on systole and nil during diastole (Fig. 11) (4,6).
 Fig. 11: In a patient presenting a clinical picture of cardiac tamponade, simultaneous recording of the electrocardiogram (ECG), right atrial pressure (OD), pericardial pressure (P), esophageal pressure (Oe), and pleural pressure (Pl). Note the virtual equalization of the pericardial and right atrial pressures, although the latter is slightly higher on ventricular systole (between the R-wave peak and the T-wave of the ECG). The recording can also be used to check that measurement of esophageal pressure gives a good indication of pleural pressure. |
The dimensions of the cardiac chambers are reduced. This reduction in size affects the left chambers (7) but is more marked in the right atrium, which may even collapse on diastole (Fig. 2 , Film 2), and at the right ventricle, where collapse is also sometimes seen (Fig. 7, Fig. 12) (8-11).
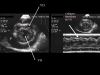 Fig. 12: Short-axis examination of the right (VD) and left (VG) ventricles in a patient presenting pericardial effusion (P). The diastolic collapse of the RV is visible both on the two-dimensional view (left) and in M-mode (right). |
The respiratoryseptal movement is abnormally accentuated, because of flattening and a shift towards the left ventricular chamber on inspiration, and bulging towards the right ventricular chamber on expiration (12) (Fig. 13, Film 4, Film 5).
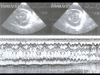 Fig. 13: Short-axis examination of the right (VD) and left (VG) ventricles in a patient presenting pericardial effusion (P). The two-dimensional examination (top) reveals septal shift, increase in RV size, and reduction in LV size, which occur on inspiration (right), compared to expiration (left).These respiratory variations are particularly well demonstrated by M-mode examination (bottom) which shows the cyclic respiratory shift of the interventricular septum (IVS) . Fig. 13: Short-axis examination of the right (VD) and left (VG) ventricles in a patient presenting pericardial effusion (P). The two-dimensional examination (top) reveals septal shift, increase in RV size, and reduction in LV size, which occur on inspiration (right), compared to expiration (left).These respiratory variations are particularly well demonstrated by M-mode examination (bottom) which shows the cyclic respiratory shift of the interventricular septum (IVS) . |
The aortic and pulmonary flow velocities studied by Doppler echocardiography have an appearance highly superimposable on that of the pulses. The inspiration is accompanied by a reduction in aortic flow velocity which is restored on expiration, whereas the reverse is true for the pulmonary flow velocity (Fig. 14, Fig. 15, Film 6) (13).
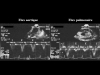 Fig. 14: Recording of aorta (left) and pulmonary artery (right) Doppler flow in a patient presenting cardiac tamponade. Note the phase contrast of these flows with an inspiratory decrease in aorta flow and an expiratory reduction in pulmonary flow. This phase contrast is the same as that seen on the invasive recording of systemic and pulmonary pulses presented in Fig. 8 and Fig. 9. |
 Fig. 15: Appearance of the four valve flows detected by Doppler in a patient in sinus rhythmpresenting cardiac tamponade. Note the inspiratory increase in right flows (tricuspid and pulmonary) and the inspiratory decrease in left flows (mitral and aortic). |
The mitral and tricuspidflow velocities have a particular Doppler appearance, with reduction of the normally predominant E-wave and preponderance of the A-wave, which is normally secondary. Furthermore, the respiratory variation of these flow velocities is abnormally marked, with accentuated reduction of the mitral flow on inspiration and accentuated reduction of the tricuspid flow on expiration (Fig. 15, Fig. 16, Fig. 17)(4,13 ).
 Fig. 16: Appearance of mitral Doppler flow in a patient in sinus rhythmpresenting cardiac tamponade, illustrating deficient left ventricular relaxation. The E-wave (rapid filling) is reduced, while the A-wave induced by atrial systole is predominant. An identical appearance of deficient right ventricular relaxation is observed at the tricuspid valve, as illustrated in Fig. 16. Fig. 16: Appearance of mitral Doppler flow in a patient in sinus rhythmpresenting cardiac tamponade, illustrating deficient left ventricular relaxation. The E-wave (rapid filling) is reduced, while the A-wave induced by atrial systole is predominant. An identical appearance of deficient right ventricular relaxation is observed at the tricuspid valve, as illustrated in Fig. 16. |
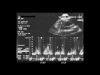 Fig. 17: Appearance of the tricuspid Doppler flow in a patient in sinus rhythmpresenting cardiac tamponade, illustrating deficient right ventricular relaxation. The E-wave (rapid filling) is reduced, while the A-wave induced by atrial systole is predominant. Note the respiratory variations in anterograde tricuspid flow, which are responsible for right ventricular diastolic filling. The flow is greatly reduced during expiration (EXP) and is restored during inspiration (INSP). Fig. 17: Appearance of the tricuspid Doppler flow in a patient in sinus rhythmpresenting cardiac tamponade, illustrating deficient right ventricular relaxation. The E-wave (rapid filling) is reduced, while the A-wave induced by atrial systole is predominant. Note the respiratory variations in anterograde tricuspid flow, which are responsible for right ventricular diastolic filling. The flow is greatly reduced during expiration (EXP) and is restored during inspiration (INSP). |
Inferior caval flow studied by Doppler, where necessary using the subhepatic veins whose axis is more favorable, always comprises an anterograde systolic flow. But the anterograde diastolic flow is abolished and replaced by a reflux (Fig. 18, Film 7)(6).
 Fig. 18: Appearance of inferior vena caval flow, recorded by Doppler echocardiography of the subhepatic vein. Note the inspiratory accentuation of the systolic wave (S), the expiratory disappearance of the diastolic wave (D), which reappears on inspiration, and the occurrence of a significant retrograde wave (directed upwards) on expiration. Fig. 18: Appearance of inferior vena caval flow, recorded by Doppler echocardiography of the subhepatic vein. Note the inspiratory accentuation of the systolic wave (S), the expiratory disappearance of the diastolic wave (D), which reappears on inspiration, and the occurrence of a significant retrograde wave (directed upwards) on expiration. |
TREATMENT Tamponade constitutes a real emergency and fluid removal is initiated immediately, using echocardiography to guide pericardial puncture (Film 8, Film 9, Film 10). Fluid removal can then be completed by surgical drainage . REFERENCES 
|
|
|
| |
|

